A Process Occurs In Which A System Potential Energy Decreases
A process occurs in which a system potential energy decreases. A thermodynamic system undergoes a process in which its internal energy decreases by 500 J. V 2 16x10-15 J 835x10-26 kg 192x10 10 J v 14x10 5 ms 3 marks Note. Because energy is conserved this energy must be released to the surroundings as thermal kinetic energy.
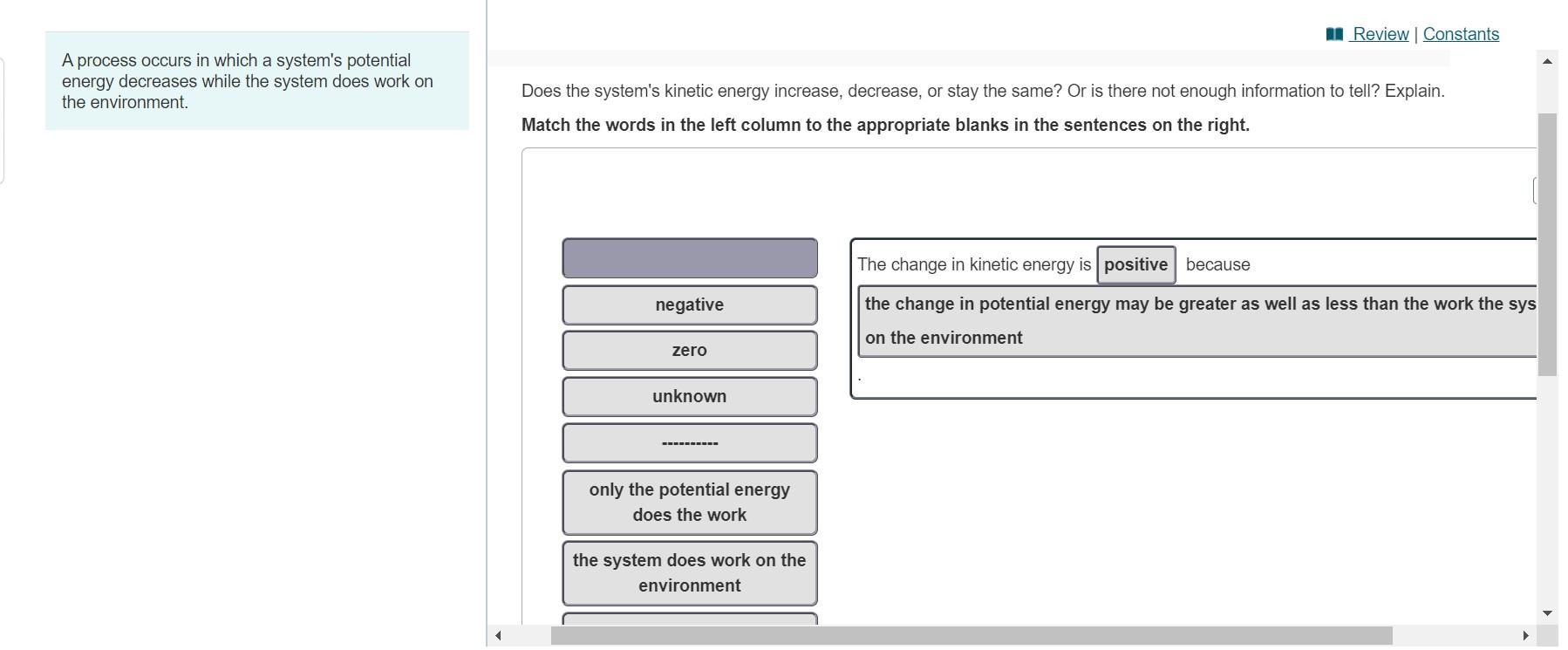
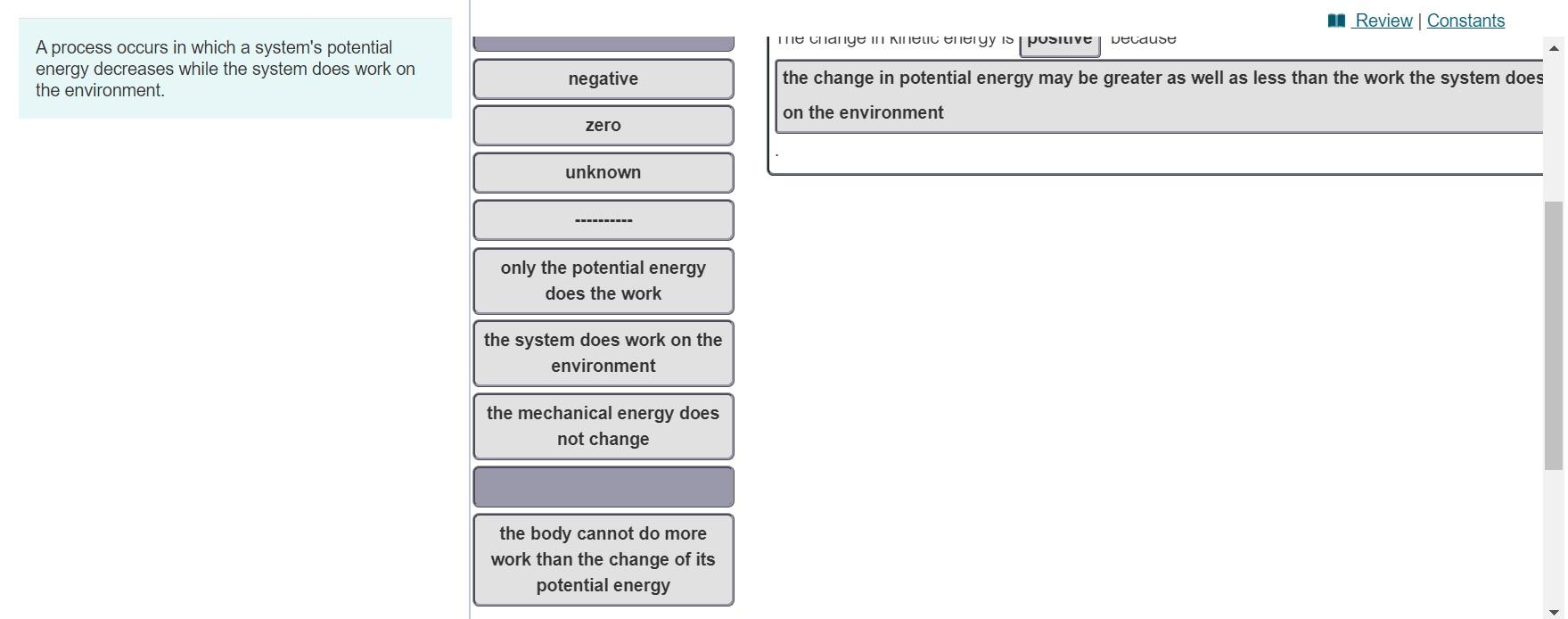
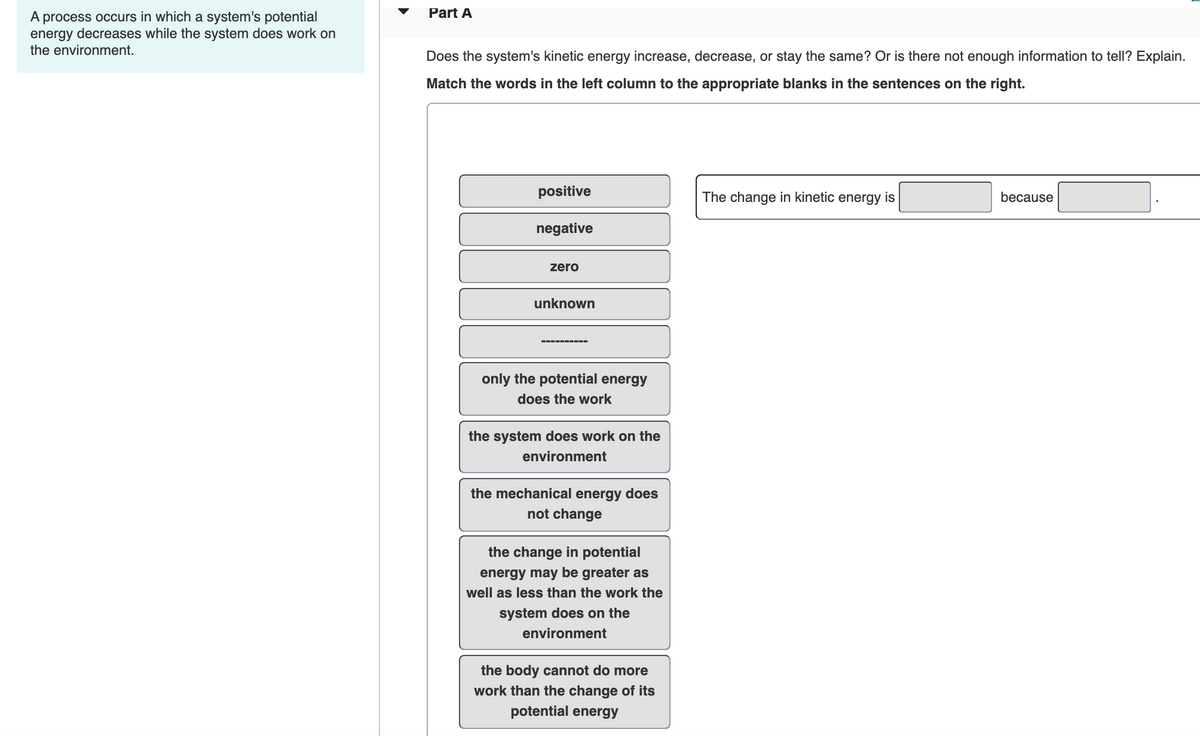
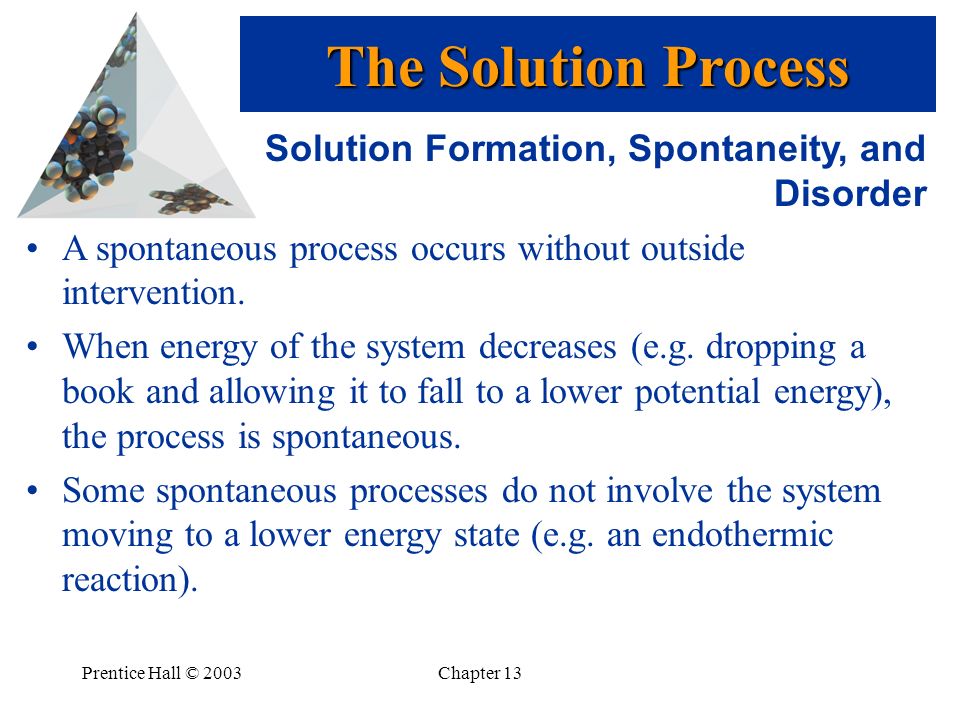
Since potential energy decreases in making bonds bond formation occurs. Once started no external actions is necessary to make the process continue. A process occurs in which a systems potential energy decreases while the system does work on the environment.
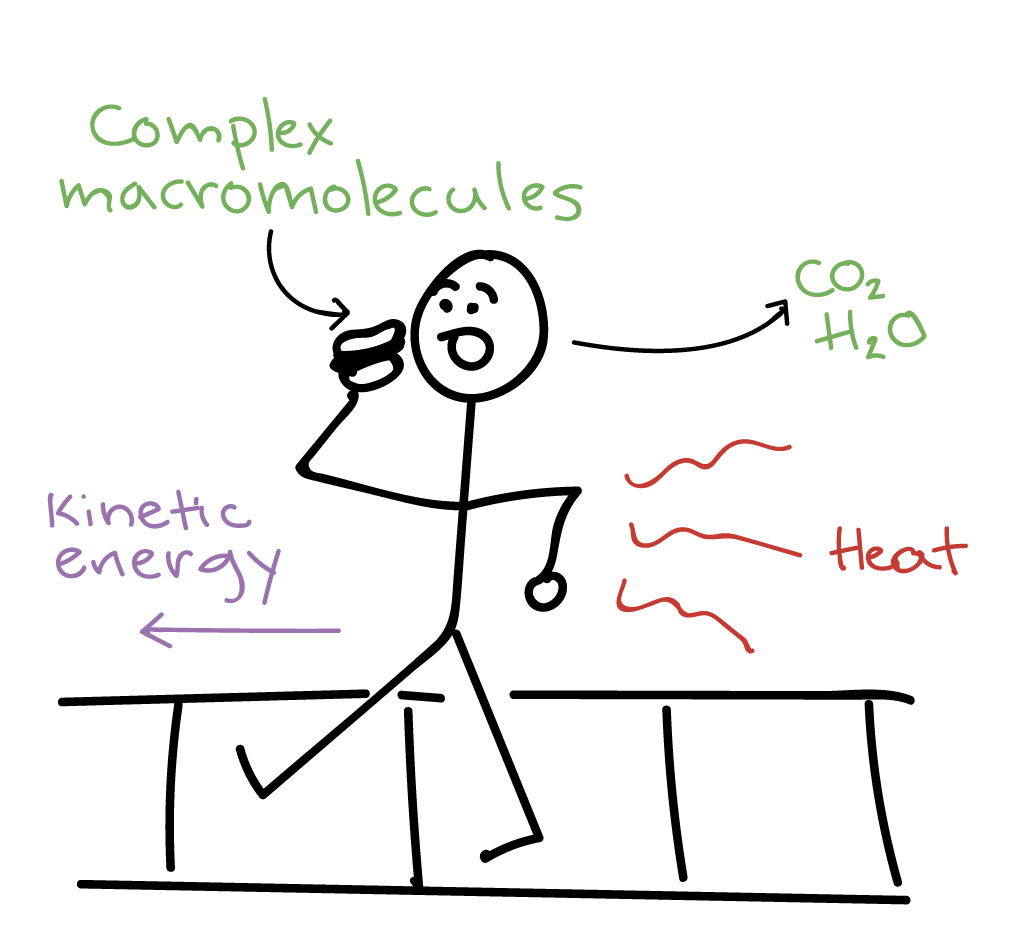
Thus the Gibbs energy enthalpy energy and entropy. If PE would decrease in breaking bonds that would also occur. While energy may change in form heat work etc and be exchanged between the system surroundings - the total energy remains constant.
That the energy of the universe is constant. 2The graph below represents the relationship between time and temperature as heat is added at a constant rate to a sample of a substance. Thermal energy was gained by the system and the surrounding air.
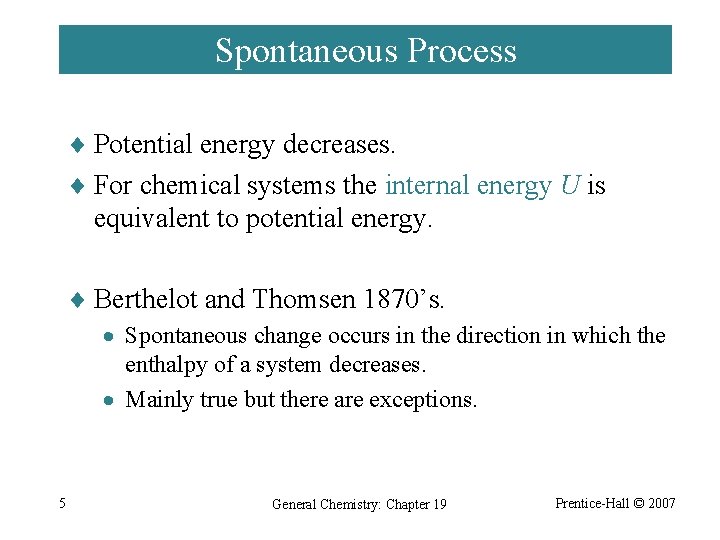
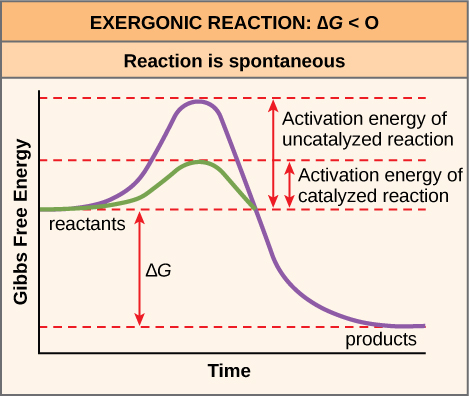
Occurs by blocking the production of an enzyme. Spontaneous Process Potential energy decreases. 2015 Pearson Education Inc.
A process occurs in which a systems potential energy decreases while the system does work on the environment. Take care to distinguish between velocity v and voltage V. The systems entropy decreases and the total entropy of the universe decreases.
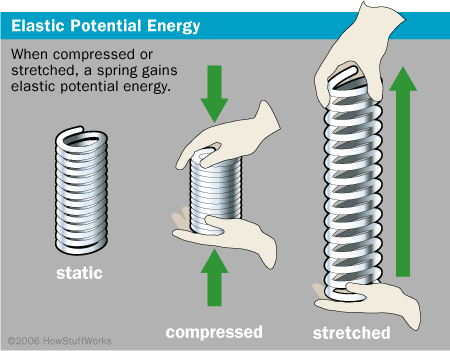
A process occurs in which a systems potential energy increases while the environment. In this case the potential energy of the system is given by k-e-er where r is the distance between the axes.
The proton gains kinetic energy equal to the potential energy lost.
The systems entropy increases and the total entropy of the universe decreases. BThe potential energy of the particles decreases. If at the same time 220 J of work is done on the system find the thermal energy transferred to or from it. Spontaneous Process Potential energy decreases. DThe average kinetic energy of the particles decreases. The Meaning of Spontaneous Change Spontaneous Process A process that occurs in a system left to itself. Occurs when a substance binds to an enzyme at a site away from the active site. In general any process in which the enthalpy or energy decreases is favorable to a decrease in G and any process in which the entropy goes up is also favorable to a decrease in G. A thermodynamic system undergoes a process in which its internal energy decreases by 300J.
Thus the Gibbs energy enthalpy energy and entropy. Heat evolved by the system is negative. A only the potential energy does the work b the system does work on the environment. Its the other way around. A thermodynamic process occurs. Thermal energy was gained by the system and the surrounding air. Work is dissipated in this process.




























Post a Comment for "A Process Occurs In Which A System Potential Energy Decreases"